Looking at https://zoom.earth/maps/pressure/
Given the much lower temperatures at the poles, I would expect the pressure to be (much) higher.
I’m reading here and there that air pressure at the antarctic is low because of its high altitude, but these maps show (I presume?) MSLP?
Low temperatures are associated with lower barometric pressure. Temperature and air pressure are proportional. You might be confusing density with air pressure?
The way it is taught in many Earth Science text books, is that if you have two similar volumes of air, but the first is colder than the second, the first will have a higher density, and therefore a larger mass, and therefore a higher air pressure (e.g. at the surface).
Following this logic, you expect higher pressure in colder areas, as long as the volume of air (the height of the air column) is the same. I think the answer to my question has to do with the latter: the atmosphere is less thick at the poles. As a result, despite the much lower temperatures, the air pressure at the poles is generally lower than (for example) at the subtropical highs.
the complication is that in meteorology, the volume of gas does not remain the same! if you’re changing the mass, temperature, density, and pressure of a parcel of air, you definitely can’t assume that the volume is constant
it’s good to use a different ideal gas equation, instead of PV = nRT (pressure x volume = n * R * Temperature)
we meteorologists tend to stick to unit masses, and use: Pressure = density ×R×T, instead
i.e. when temperature decreases, pressure decreases
But clearly, on a global scale, the opposite is true? As in, for example, the ITCZ is located at the subsolar point, where the planet receives the most irradiation, and this is an area of low pressure (and convection)? High temperatures -> low pressure.
The ITCZ is an interesting case to use here! You’re right that it’s the thermal equator and has low pressure, but you’ve gotta consider convection and wind direction too (i.e. the whole Hadley cell). Convection (caused by solar heating) causes low pressure too, and pressure is often relative.
When you’re thinking about stuff on a global scale you’ve always gotta consider the global atmospheric circulation
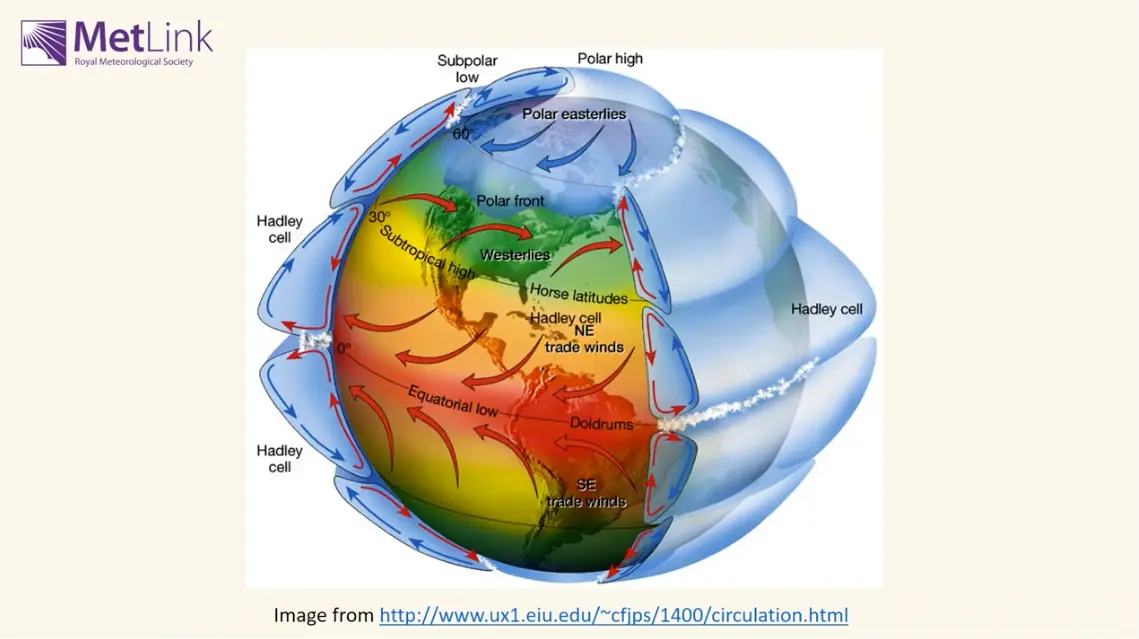
There’s a lot of good explanation in the link I sent - it’s tricky trying to consider all the variables together, but I would say that variations in density (latitudinally, at least - unless you want to start talking about hydrostatic balance!) doesn’t account for the variation in pressure or temperature.
Also, the formula Pressure = density x R x T doesn’t show that pressure decreases as temperature decreases, since a temperature decrease also increase the density?


