Deuterium UV lamp for my DIY absorption spectrometer.
Took this photo today and I thought it looked pretty cool, so I wanted to share!
This looks so cool!! Can you ELI5 what this does?
For sure! I can try.
One common way of making light is to create a glass chamber with some conductive metal wires inside. In some lamp types you have a continuous wire, and in some lamps you have gaps in the wires that the electricity has to jump through when a current flows. The air is removed and some specific gas is added into the chamber. The flow of electricity can heat up the metal wires and the gas, bombard the gas with electrons, and in some cases even drive a chemical reaction cycle. In these processes the gas and wires gain energy, and this energy is then released as a mixture of light particles with different energies.
Different lamps produce different types of light and they are useful in different contexts. For example, in the past the common light bulbs used a tungsten filament with small amounts of halogens to produce visible light. These type of lamps emit light in the visible and infra-red ranges, but they do not emit a lot of ultra-violet light. In spectroscopy, often we want to measure how the ultraviolet light is absorbed.
The “deuterium arc lamp” is a type of lamp that uses diatomic deuterium (heavy hydrogen) as the gas. The reason is that this gas happens to show a strong and “broad” emission in the ultraviolet region, meaning that it will produce a mixture of light particles with energies that cover the ultraviolet range of the spectrum. Here is a comparison of the light emitted by a standard tungsten-halogen lamp versus a deuterium lamp:
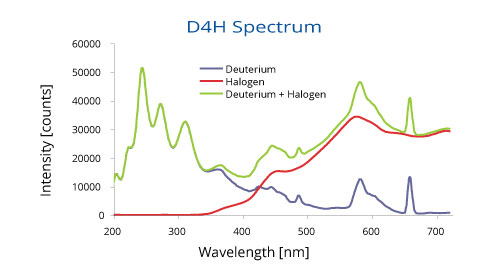
A common approach used in spectroscopy is to combine the light from both a tungsten halogen lamp and a deuterium lamp to obtain light that covers from the ultraviolet range all the way into the infra-red.